What is molecular weight of a compound?
Molecular weight of a compound is the sum of atomic masses of each atom in the molecule.
Molecular weight of a compound can be found out by following these straightforward steps:
-
Find out the molecular formula of the compound.
-
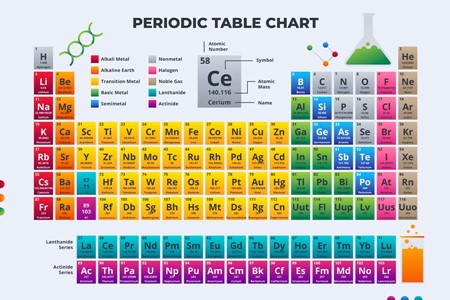
Using the periodic table find out the atomic mass for each compound.
-
After you have found out the atomic mass for each atom multiply that with the number of atoms of each element in a compound.
-
Finally add all the elements of the compound together.
You will arrive at the molecular weight of the compound.
If the steps mentioned above was not clear to you then don’t worry.Here is a simple illustration to explain the process.
Let us find out the molecular weight of Sodium Oxalate.
The first step is to find out the molecular formula of Sodium Oxalate which is Na2C2O4
Next using the periodic table find out the atomic masses of each element in the molecular formula.
There are three atoms in molecular formula of Sodium Oxalate.
Sodium Atom (Na)
Carbon Atom (C)
Oxygen Atom (O)
The atomic weight for each of these atoms is as follows:
Na = 22.989769
C = 12.011
O = 15.999
Next determine the subscripts of each atom in the molecule.
(Subscripts tells us how many types of each atom is present in a molecule)
The number below each atom is the subscript for that atom.
Thus, in the molecular formula for Sodium Oxalate. There are:
2 subscripts of Na
2 subscripts of C
4 subscripts of O
After determining the number of subscripts of each atom multiply it with the atomic weight of that atom.
Molecular mass of Sodium Oxalate = (No. of Atoms of Na * Atomic Weight of Na) + (No. of Atoms of C * Atomic Weight of C) + ( No. of Atoms of O * Atomic Weight of O)
Molecular mass of Sodium Oxalate = (2 * 22.99) + (2*12.01) + (4 * 16)
Molecular mass of Sodium Oxalate =134 g
Let us find out molecular weight for another compound. For eg. Di Ammonium Phosphate.
The molecular formula of Di Ammonium Phosphate is (NH4)2HPO4
The molecular mass of each atom is as follows:
NH4 = 18.04
H =1.00784
P=30.97
O=15.999
Molecular mass of Di Ammonium Phosphate = (18.04*2) + 1.01 + 30.97 + (16*4)
Molecular mass of Di Ammonium Phosphate = 36.08+1.01+30.97+64
Molecular mass of Di Ammonium Phosphate =132.06 g
In conclusion, the molecular weight of a compound is a crucial parameter that quantifies the total mass of its constituent atoms. By following the straightforward steps outlined above, we can calculate the molecular weight of any compound with ease. First, determine the molecular formula and identify the atomic masses of each element from the periodic table. Next, find the number of atoms for each element by observing the subscripts in the molecular formula. Finally, multiply the atomic masses with their respective atom counts and sum them up to find the molecular weight of the compound.